【引用本文】肝癌新辅助治疗中国专家共识协作组,中国研究型医院学会消化外科专业委员会,中国抗癌协会肝癌专业委员会.肝癌新辅助治疗中国专家共识2023版)[J].中华外科杂志,2023,61(12):1-11.
肝细胞癌(简称肝癌)术后复发是影响患者治疗效果改善的主要因素,新辅助治疗是降低术后复发、延长患者生存的有效治疗措施,但目前肝癌新辅助治疗尚无公认的有效方案。近年来,随着以靶向、免疫检测点抑制剂为代表的系统抗肿瘤药物的进步,以及肝癌局部治疗方法的改进,这些治疗方案在肝癌新辅助治疗领域有了初步有效的探索。肝癌新辅助治疗中国专家共识协作组在中国研究型医院学会消化外科专业委员会和中国抗癌协会肝癌专业委员会的组织领导下,经过多次讨论和修订,制定了《肝癌新辅助治疗中国专家共识(2023版)》,旨在结合中国肝癌诊疗特点,为术前肝癌治疗决策提供有针对性的指导性建议,同时进一步规范新辅助治疗的实施路径。
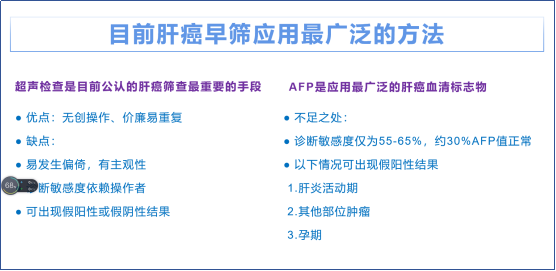
原发性肝癌是全球第六大常见癌症和第三大癌症死亡原因,2020年全球新增原发性肝癌约90万例,新增原发性肝癌相关死亡约 83万例。其中中国原发性肝癌新增病例约41万例(占比 45.3%),新增死亡约39万例(占比47.1%),均居全球首位[1]。肝细胞癌(简称肝癌)是原发性肝癌最常见的病理学类型,在我国约占原发性肝癌病例的90%[2]。外科治疗是可切除肝癌患者的首选治疗方法。近年来肝癌患者肝切除术后5年总体生存率已提高至60.0%,但肝癌术后复发率高,尤其是中国肝癌分期(China liver cancer staging,CNLC)Ⅱb和Ⅲa期的可切除肝癌患者,其术后1年复发率超过55%,Ⅰb和Ⅱa期的肝癌患者复发率达到了32.4%和45.7%[3]。降低术后复发风险、延长患者生存时间,是肝癌新辅助治疗的主要目标,但目前尚未形成公认的标准化治疗范式。
为了进一步厘清新辅助治疗的概念,掌握治疗指征、明确手术时机、选择最佳药物治疗方案,南京医科大学第一附属医院从2019年起开展了一系列肝癌新辅助治疗的临床和基础研究[4, 5, 6],在此基础上,中国研究型医院学会消化外科专业委员会和中国抗癌协会肝癌专业委员会组织相关专家,于 2023年3月启动了《肝癌新辅助治疗中国专家共识(2023版)》的制定工作。本共识旨在结合中国肝癌诊疗特点,为术前新辅助治疗决策提供指导性建议,同时进一步规范新辅助治疗的实施路径。
本共识中的循证医学证据等级评价参照了证据评价与推荐意见分级、制定和评价(grading of recommendations,assessment,development and evaluation,GRADE)分级[7]和《牛津循证医学中心分级2011版》(表1),专家推荐强度主要参照GRADE对推荐意见分级的指导原则[8],并结合ASCO指南的分级方案[9]对推荐意见分级进行了部分修改。本文中将推荐意见分为A(强推荐)、B(中等程度推荐)和C(弱推荐)三个等级(表2)。推荐强度A表示专家组对该推荐意见反映了最佳临床实践有很强的信心,并认为目标人群都应该采纳该推荐意见。推荐强度B表示专家组对该推荐意见反映了最佳临床实践有中等程度的信心,多数目标人群应采纳该推荐意见,执行过程中应考虑医患共同决策。推荐强度C表示专家组对该推荐意见反映了最佳临床实践有一定的信心,应该有条件地应用于目标人群,强调根据患者的价值偏好进行医患共同决策。
表1 牛津循证医学中心 2011 版证据等级内容

表2 证据推荐强度的定义描述

一、共识制定方法学
二、肝癌新辅助治疗的概念、适用人群、治疗周期
三、MDT团队在肝癌新辅助治疗中的必要性
四、肝癌新辅助治疗方法
五、肝癌新辅助治疗的有效性评估指标
六、肝癌新辅助治疗的不良反应
七、肝癌术后辅助治疗
八、新辅助治疗的终止时机及治疗失败后的后续治疗
九、结语
《肝癌新辅助治疗中国专家共识(2023版)》编审委员会成员
参考文献
(在框内滑动手指即可浏览)
[1]SungH,FerlayJ,SiegelRL,et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J].CA Cancer J Clin,2021,71(3):209-249. DOI: 10.3322/caac.21660.
[2]CongWM,DongH,TanL,et al. Surgicopathological classification of hepatic space-occupying lesions: a single-center experience with literature review[J].World J Gastroenterol,2011,17(19):2372-2378. DOI: 10.3748/wjg.v17.i19.2372.
[3]夏永祥,张峰,李相成,等. 原发性肝癌10 966例外科治疗分析[J].中华外科杂志,2021,59(1):6-17. DOI: 10.3760/cma.j.cn112139-20201110-00791.
[4]XiaY,TangW,QianX,et al. Efficacy and safety of Camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm,open label,phase Ⅱ clinical trial[J]. J Immunother Cancer,2022,10(4):e004656. DOI: 10.1136/jitc-2022-004656.
[5]LiQ,ZhangL,YangQ,et al. Thymidine kinase 1 drives hepatocellular carcinoma in enzyme-dependent and-independent manners[J].Cell Metab,2023,35(6):912-927. DOI: 10.1016/j.cmet.2023.03.017.
[6]LiQ,ZhangL,YouW,et al. PRDM1/BLIMP1 induces cancer immune evasion by modulating the USP22-SPI1-PD-L1 axis in hepatocellular carcinoma cells[J].Nat Commun,2022,13(1):7677. DOI: 10.1038/s41467-022-35469-x.
[7]BalshemH,HelfandM,SchünemannHJ,et al. GRADE guidelines: 3. Rating the quality of evidence[J]. J Clin Epidemiol,2011,64(4):401-406. DOI: 10.1016/j.jclinepi.2010.07.015.
[8]AndrewsJC,SchünemannHJ,OxmanAD,et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation′s direction and strength[J]. J Clin Epidemiol,2013,66(7):726-735. DOI: 10.1016/j.jclinepi.2013.02.003.
[9]American Society of Clinical Oncology. ASCO Measures Methodology Manual [S/OL].[2021-12-09]. https://www.asco.org/sites/new-www.asco.org/files/content-files/advocacy-and-policy/documents/Guidelines-Methodology-Manual_0.pdf.
[10]国家卫生健康委办公厅. 原发性肝癌诊疗指南(2022年版)[J]. 中华外科杂志,2022,60(4):273-309.DOI: 10.3760/cma.j.cn112139-20220217-00068.
[11]YangP,QiuJ,LiJ,et al. Nomograms for pre-and postoperative prediction of long-term survival for patients who underwent hepatectomy for multiple hepatocellular carcinomas[J].Ann Surg,2016,263(4):778-786. DOI: 10.1097/SLA.0000000000001339.
[12]LeiZ,LiJ,WuD,et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria[J].JAMA Surg,2016,151(4):356-363. DOI: 10.1001/jamasurg.2015.4257.
[13]张珈玮,关连越,鄂长勇,等. 血清异常凝血酶原在肝细胞肝癌临床应用中的价值[J].中华外科杂志,2020,58(10):776-781.DOI:10.3760/cma.j.cn112139-20200313-00219.
[14]JiGW,ZhuFP,XuQ,et al. Radiomic features at contRASt-enhanced CT predict recurrence in early stage hepatocellular carcinoma: a multi-institutional study[J].Radiology,2020,294(3):568-579. DOI: 10.1148/radiol.2020191470.
[15]LlovetJM,VillanuevaA,MarreroJA,et al. Trial design and endpoints in hepatocellular carcinoma: AASLD Consensus Conference[J].Hepatology,2021,73Suppl 1:S158-S191. DOI: 10.1002/hep.31327.
[16]BertaccoA,VitaleA,MescoliC,et al. Sorafenib treatment has the potential to downstage advanced hepatocellular carcinoma before liver resection[J].Per Med,2020,17(2):83-87. DOI: 10.2217/pme-2018-0114.
[17]KimTS,KimJH,KimBH,et al. Complete response of advanced hepatocellular carcinoma to sorafenib: another case and a comprehensive review[J].Clin Mol Hepatol,2017,23(4):340-346. DOI: 10.3350/cmh.2016.0070.
[18]IrtanS,Chopin-LalyX,RonotM,et al. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection[J].Liver Int,2011,31(5):740-743. DOI: 10.1111/j.1478-3231.2010.02441.x.
[19]KasebAO,HasanovE,CaoH,et al. Perioperative nivolumab monotherapy versus nivolumab plus Ipilimumab in resectable hepatocellular carcinoma: a randomised,open-label,phase 2 trial[J].Lancet Gastroenterol Hepatol,2022,7(3):208-218. DOI: 10.1016/S2468-1253(21)00427-1.
[20]KasebAO,VenceL,BlandoJ,et al. Immunologic correlates of pathologic complete response to preoperative immunotherapy in hepatocellular carcinoma[J].Cancer Immunol Res,2019,7(9):1390-1395. DOI: 10.1158/2326-6066.CIR-18-0605.
[21]中国抗癌协会肝癌专业委员会转化治疗协作组. 肝癌转化治疗中国专家共识(2021版)[J].中华消化外科杂志,2021,20(6):600-616.DOI: 10.3760/cma.j.cn115610-20210512-00223.
[22]ClearyJM,TanabeKT,LauwersGY,et al. Hepatic toxicities associated with the use of preoperative systemic therapy in patients with metastatic colorectal adenocarcinoma to the liver[J].Oncologist,2009,14(11):1095-1105. DOI: 10.1634/theoncologist.2009-0152.
[23]LiC,WangMD,LuL,et al. Preoperative transcatheter arterial chemoembolization for surgical resection of huge hepatocellular carcinoma (≥10 cm): a multicenter propensity matching analysis[J].Hepatol Int,2019,13(6):736-747. DOI: 10.1007/s12072-019-09981-0.
[24]MajnoPE,AdamR,BismuthH,et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis[J].Ann Surg,1997,226(6): 688-703.DOI: 10.1097/00000658-199712000-00006.
[25]LiN,FengS,XueJ,et al. Hepatocellular carcinoma with main portal vein tumor thrombus: a comparative study comparing hepatectomy with or without neoadjuvant radiotherapy[J].HPB (Oxford),2016,18(6):549-556. DOI: 10.1016/j.hpb.2016.04.003.
[26]SiddiqueO,YooER,PerumpailRB,et al. The importance of a multidisciplinary approach to hepatocellular carcinoma[J].J Multidiscip Healthc,2017, 10:95-100. DOI: 10.2147/JMDH.S128629.
[27]AlabrabaE,JoshiH,BirdN,et al. Increased multimodality treatment options has improved survival for Hepatocellular carcinoma but poor survival for biliary tract cancers remains unchanged[J].Eur J Surg Oncol,2019,45(9):1660-1667. DOI: 10.1016/j.ejso.2019.04.002.
[28]CharriereB,MuscariF,MaulatC,et al. Outcomes of patients with hepatocellular carcinoma are determined in multidisciplinary team meetings[J].J Surg Oncol,2017,115(3):330-336. DOI: 10.1002/jso.24500.
[29]SangiovanniA,TrioloM,IavaroneM,et al. Multimodality treatment of hepatocellular carcinoma: how field practice complies with international recommendations[J].Liver Int,2018,38(9):1624-1634. DOI: 10.1111/liv.13888.
[30]ZhouWP,LaiEC,LiAJ,et al. A prospective,randomized,controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma[J].Ann Surg,2009,249(2):195-202. DOI: 10.1097/SLA.0b013e3181961c16.
[31]ShiHY,WangSN,WangSC,et al. Preoperative transarterial chemoembolization and resection for hepatocellular carcinoma: a database analysis of long-term outcome predictors[J].J Surg Oncol,2014,109(5):487-493. DOI: 10.1002/jso.23521.
[32]ZhangYF,GuoRP,ZouRH,et al. Efficacy and safety of preoperative chemoembolization for resectable hepatocellular carcinoma with portal vein invasion: a prospective comparative study[J].Eur Radiol,2016,26(7):2078-2088. DOI: 10.1007/s00330-015-4021-8.
[33]ZhuHD,LiHL,HuangMS,et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001)[J].Signal Transduct Target Ther,2023,8(1):58. DOI: 10.1038/s41392-022-01235-0.
[34]GuoC,ZhangJ,HuangX,et al. Preoperative Sintilimab plus transarterial chemoembolization for hepatocellular carcinoma exceeding the Milan criteria: a phase Ⅱ trial[J].Hepatol Commun,2023,7(3):e0054. DOI: 10.1097/HC9.0000000000000054.
[35]WeiW,LiS,ZhaoR,et al. Neoadjuvant hepatic arterial infusion chemotherapy with FOLFOX could improve outcomes of resectable BCLC stage A/B hepatocellular carcinoma patients beyond Milan criteria: a multi-center,phase 3,randomized,controlled clinical trial[G].J Clin Oncol,2023,41(16_suppl):4023-4023. DOI: 10.1200/JCO.2023.41.16_suppl.4023.
[36]PanY,MeiJ,ChenJ,et al. Comparison between portal vein perfusion chemotherapy and neoadjuvant hepatic arterial infusion chemotherapy for resectable intermediate to advanced stage hepatocellular carcinoma[J].Ann Surg Oncol,2022,29(3):2016-2029. DOI:10.1245/s10434-021-10903-4.
[37]WeiX,JiangY,ZhangX,et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized,open-label,multicenter controlled study[J].J Clin Oncol,2019,37(24):2141-2151. DOI: 10.1200/JCO.18.02184.
[38]WuF,ChenB,DongD,et al. Phase 2 evaluation of neoadjuvant intensity-modulated radiotherapy in centrally located hepatocellular carcinoma: a nonrandomized controlled trial[J].JAMA Surg,2022,157(12):1089-1096. DOI: 10.1001/jamasurg.2022.4702.
[39]JinH,QinS,HeJ,et al. New insights into checkpoint inhibitor immunotherapy and its combined therapies in hepatocellular carcinoma: from mechanisms to clinical trials[J].Int J Biol Sci,2022,18(7):2775-2794. DOI: 10.7150/ijbs.70691.
[40]SangroB,SarobeP,Hervás-StubbsS,et al. Advances in immunotherapy for hepatocellular carcinoma[J].Nat Rev Gastroenterol Hepatol,2021,18(8):525-543. DOI: 10.1038/s41575-021-00438-0.
[41]MarronTU,FielMI,HamonP,et al. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm,open-label,phase 2 trial[J].Lancet Gastroenterol Hepatol,2022,7(3):219-229. DOI: 10.1016/S2468-1253(21)00385-X.
[42]夏永祥,张慧,张峰,等. 肝细胞癌免疫新辅助治疗的有效性和安全性分析[J].中华外科杂志,2022,60(7):688-694. DOI: 10.3760/cma.j.cn112139-20220408-00150.
[43]ZhaoM,ChenS,LiC,et al. Neoadjuvant Immune Checkpoint Inhibitors for Resectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis[J].Cancers (Basel),2023,15(3):600. DOI: 10.3390/cancers15030600.
[44]WangG,ZhuS,LiX. Comparison of values of CT and MRI imaging in the diagnosis of hepatocellular carcinoma and analysis of prognostic factors[J].Oncol Lett,2019,17(1):1184-1188. DOI: 10.3892/ol.2018.9690.
[45]ZhuXD,HuangC,ShenYH,et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations[J].Liver Cancer,2021,10(4):320-329. DOI: 10.1159/000514313.
[46]国家药品监督管理局药品审评中心.晚期肝细胞癌临床试验终点技术指导原则(2020年第44号)[S/OL].[2020-11-26]. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20201204162342100.html.
[47]TravisWD,DacicS,WistubaI,et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy[J].J Thorac Oncol,2020,15(5):709-740. DOI: 10.1016/j.jtho.2020.01.005.
[48]ValdagniR,RancatiT,FiorinoC. Predictive models of toxicity with external radiotherapy for prostate cancer: clinical issues[J].Cancer,2009,115(13Suppl):3141-3149. DOI: 10.1002/cncr.24356.
[49]LoiM,ComitoT,FranzeseC,et al. Stereotactic body radiotherapy in hepatocellular carcinoma: patient selection and predictors of outcome and toxicity[J]. J Cancer Res Clin Oncol,2021,147(3):927-936. DOI: 10.1007/s00432-020-03389-2.
[50]中国临床肿瘤学会指南工作委员会.免疫检查点抑制剂相关的毒性管理指南[M].北京:人民卫生出版社,2021.
[51]DasA,MahapatRAS,BandyopadhyayD,et al. Bleeding with vascular endothelial growth factor tyrosine kinase inhibitor: A network meta-analysis[J].Crit Rev Oncol Hematol,2021, 157:103186. DOI:10.1016/j.critrevonc.2020.103186.
[52]FinnRS,QinS,IkedaM,et al. atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma[J].N Engl J Med,2020,382(20):1894-1905. DOI: 10.1056/NEJMoa1915745.
[53]施国明,黄晓勇,任正刚,等. 肝癌免疫检查点抑制剂相关不良反应管理中国专家共识(2021版)[J].中华消化外科杂志,2021,20(12):1241-1258. DOI:10.3760/cma.j.cn115610-20211125-00594.
[54]肝癌术后辅助治疗中国专家共识协作组,中国医师协会外科医师分会,中国抗癌协会肝癌专业委员会,等. 肝癌术后辅助治疗中国专家共识(2023版)[J].中华消化外科杂志,2023,22(4):437-448. DOI:10.3760/cma.j.cn115610-20230315-00108.